![]()
Materials:

Methods:
![]()
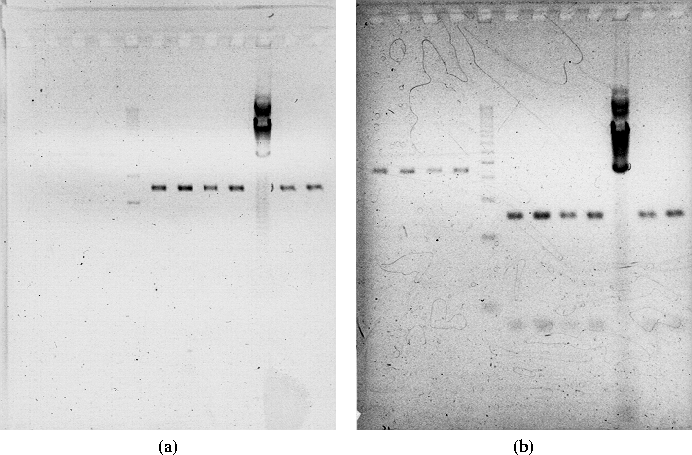
Figure 3.3: (a) Gel before restaining, showing only ladders and
pGFP(LVA) without GFP(LVA) insert. (b) Gel after staining, now
also showing pBR322 vector and GFP(LVA) insert. [Note: figs not to
scale]
Results: ( 1/29/99)
Ran the gel for 1:20 hours (see
Figure 3.3(a)) but because I forgot to add
EtBr to the gel (red) chamber, the results don't show the DNA. The
pGFP(LVA) for all samples shows as a strong band somewhere between 1kb
and 2kb. The GFP(LVA) insert is not showing. The pBR322 for all
samples appears as a very faint band, probably between 3kb and 4kb. I
pipetted ![]() EtBr into TE buffer chamber of the gel
apparatus (on red side), but that didn't really help.
EtBr into TE buffer chamber of the gel
apparatus (on red side), but that didn't really help.
I therefore restained the gel (according to protocol in Appendix E.2). Figure 3.3 (b) shows the restained gel. Now, both the pBR322 vector and the GFP(LVA) insert are visible. I excised the pBR322 into 2 tubes and the GFP(LVA) insert into 3 tubes, and added the following according to QIAEX II protocol:

Then, added ![]() QIAEX II. Then, eluted with
QIAEX II. Then, eluted with ![]() Tris-HCl, pH 8.5, centrifuged, pipetted the supernatant into clean
tube, and stored
Tris-HCl, pH 8.5, centrifuged, pipetted the supernatant into clean
tube, and stored ![]() . All DNA tubes have about
. All DNA tubes have about ![]() solution volume. The GFP(LVA) tubes should have 30ng DNA each
(
solution volume. The GFP(LVA) tubes should have 30ng DNA each
( ![]() DNA), and the pBR322 tubes should have 50ng DNA
each (
DNA), and the pBR322 tubes should have 50ng DNA
each ( ![]() DNA).
DNA).