



Next: Concentrate DNA using Microcon
Up: Protocol: Prepative Gel to
Previous: Protocol: Prepative Gel to
Materials:
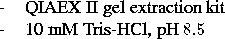
Methods:
- Excise the DNA band from the agarose gel with a clean, sharp
scalpel. Minimize the size of the gel slice by removing excess
agarose. Collect the gel into a 1.5ml microfuge tube for processing
up to 250 mg agarose.
- Weigh the gel slice in a colorless tube. Add 3 volumes of
Buffer QX 1 to 1 volume of gel for DNA fragments 100bp - 4kb.
- Resuspend QIAEX II by vortexing for 30 sec. Add QIAEX II to the
sample according to the table below and mix:

- Incubate at
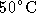 for 10 min to solubilize the
agarose and bind the DNA. Mix by vortexing every 2 min to keep QIAEX
II in suspension. Check that the color of the mixture is yellow.
for 10 min to solubilize the
agarose and bind the DNA. Mix by vortexing every 2 min to keep QIAEX
II in suspension. Check that the color of the mixture is yellow.

- Centrifuge the sample for 30 sec and carefully remove
supernatant with a pipet.
- Wash the pellet with 500
 of Buffer QX1: Resuspend
the pellet by vortexing. Centrifuge the sample for 30 sec and remove
all traces of supernatant with a pipet. This wash step removes
residual agarose contaminants.
of Buffer QX1: Resuspend
the pellet by vortexing. Centrifuge the sample for 30 sec and remove
all traces of supernatant with a pipet. This wash step removes
residual agarose contaminants.
- Wash the pellet twice with 500
 of Buffer PE:
Resuspend the pellet by vortexing. Centrifuge the sample for 30 sec
and carefully remove all traces supernatant with a pipet. These
washing steps remove residual salt contaminants.
of Buffer PE:
Resuspend the pellet by vortexing. Centrifuge the sample for 30 sec
and carefully remove all traces supernatant with a pipet. These
washing steps remove residual salt contaminants.
- Air-dry the pellet for 10-15 min or until the pellet becomes
white. For
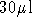 suspension, air-dry for 30 minutes.
suspension, air-dry for 30 minutes.
- To elute DNA, add
 of 10 mM Tris-Cl, pH 8.5 or
of 10 mM Tris-Cl, pH 8.5 or
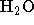 and resuspend the pellet by vortexing. For DNA fragments
and resuspend the pellet by vortexing. For DNA fragments
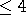 kb, incubate at room temp for 5 min. For DNA fragments
4-10 kb, incubate
kb, incubate at room temp for 5 min. For DNA fragments
4-10 kb, incubate 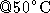 for 5 min.
for 5 min.
- Centrifuge for 30 sec. Carefully pipet the supernatant into a
clean tube. The supernatant now contains the purified DNA.
- Store the DNA





Next: Concentrate DNA using Microcon
Up: Protocol: Prepative Gel to
Previous: Protocol: Prepative Gel to
Ron Weiss
Wed Feb 10 15:48:08 EST 1999
![]()
