We set n=4. We have found that this value produces a good quality inverter.
We need to put further constraints on our concentrations to make a
useful inverter. In particular, we want the output of an inverter
to be a signal in the same analog range as the input, and we may
require the output to go through the halfway point just when the input
does. These constraints will restrict the possible values of the
parameters ![]() ,
,
![]() ,
,
![]() ,
and [B0].
,
and [B0].
Let's start by declaring that the range of signals is the interval [0, 1]. So we require that [A]=0 when [B]=1. This is a free choice, because it just determines the units that we use to measure the concentrations. We can also require that [A]=1/2 when [B]=1/2. This is an actual restriction on our transfer characteristic. Plugging these constraints into the equation (17) results in the relationships:
These leave us with exactly one free parameter for the transfer
characteristics of our inverter,
![]() .
Do we get a good
inverter for reasonable values of this parameter? The answer is yes.
.
Do we get a good
inverter for reasonable values of this parameter? The answer is yes.
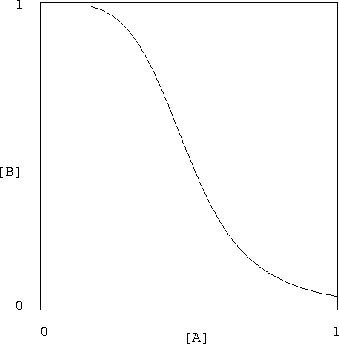
Figure 1 shows that the relationship in
equation (17) between the input and output concentrations yields
the classic transfer curve of a good digital inverter. (Here n=4and
![]() .) Note, in particular, the low gain for high
and low input concentrations, separated by a relatively high-gain
transition region. This nonlinearity is the essence of digital gates,
and forms the basis for effectively rejecting small variations in the
input signals--that is, for attenuating the input noise.
.) Note, in particular, the low gain for high
and low input concentrations, separated by a relatively high-gain
transition region. This nonlinearity is the essence of digital gates,
and forms the basis for effectively rejecting small variations in the
input signals--that is, for attenuating the input noise.
 |
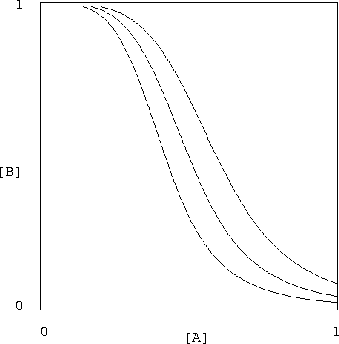
It is encouraging that the proposed inverter shows promising low
sensitivity to variations in the chemical rate constants. The
sets of curves shown in figures 2
and 3 display the results of halving and doubling of
each of the constants ![]() ,
,
![]() in equation (17), while
holding
in equation (17), while
holding
![]() constant. Of course, changing these constants
violates our constraints (equations (18,19)) but the
resulting system is still quite serviceable.
constant. Of course, changing these constants
violates our constraints (equations (18,19)) but the
resulting system is still quite serviceable.
 |
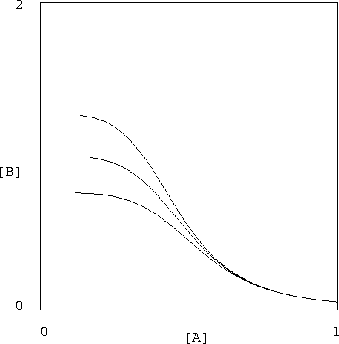 |
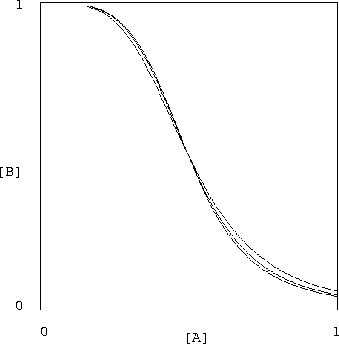
What is even more spectacularly encouraging is that the value of
![]() can be varied over a huge range without making this
inverter unusable. In figure 4 we see that
varying
can be varied over a huge range without making this
inverter unusable. In figure 4 we see that
varying
![]() over the entire range [1, 50] has almost no
effect on the characteristic shape of our inverter.
over the entire range [1, 50] has almost no
effect on the characteristic shape of our inverter.
 |
Of course, this is still useless unless the kinetic constants for biologically feasible reactions are within these ranges. We have not yet done this analysis, but we hope to have results to report shortly.