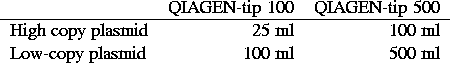Next: Protocol: Making Plates
Up: Protocol: Prep to Extract
Previous: Qiagen-tip 20 Miniprep
Materials:

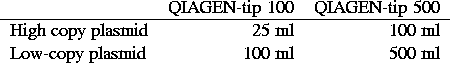
Note: amounts denoted by x/y are for QIAGEN-tip 100 / QIAGEN-tip
500.
Methods:
- Chill Buffer P3 on ice (also P1?).
- For each bacterial strain, use appropriate amount of culture
according to above table.
- Harvest the bacterial cells by centrifugation at 6000
 g
for 15 min
g
for 15 min  . Remove all traces of supernatant by inverting
the open centrifuge tube until all medium has been drained.
. Remove all traces of supernatant by inverting
the open centrifuge tube until all medium has been drained.
- Resuspend the bacterial pellet in 4/10 ml of buffer P1. Vortex
until there are no more clumps. Pipet the contents of the third tube
into each of the other two tubes.
- Add 4/10 ml of Buffer P2, mix gently but thoroughly by inverting 4-6
times, and incubate at room temprerature for 5 min. Do not vortex.
Do not allow lysis reaction to proceed for more than 5 min. Close
Buffer P2 bottle immediately.
- Add 4/10 ml of chilled Buffer P3, mix immediately but gently by
inverting 4-6 times, and incubate on ice for 15/20 min.
- Mix the sample again, before loading the centrifuge. Centrifuge
at
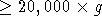 for 30 min
for 30 min  . Remove supernatant
containing plasmid DNA promptly. After centrifugation, the
supernatant should be clear.
. Remove supernatant
containing plasmid DNA promptly. After centrifugation, the
supernatant should be clear.
- Re-centrifuge the supernatant at
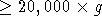 for 15
min
for 15
min  . Remove supernatant containing plasmid DNA promptly.
. Remove supernatant containing plasmid DNA promptly.
- Equilibrate a QIAGEN-tip 100/500 by applying 4/10 ml Buffer QBT, and
allow the column to empty by gravity flow.
- Apply the supernatant of plasmid DNA to the QIAGEN-tip and allow
it to enter the resin by gravity flow. The supernatant should be
loaded onto the QIAGEN-tip promptly. If it becomes cloudy, it must be
recentrifuged.
- Wash the QIAGEN-tip with
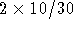 ml Buffer QC. Allow
Buffer QC to move through the QIAGEN-tip by gravity flow.
ml Buffer QC. Allow
Buffer QC to move through the QIAGEN-tip by gravity flow.
- Elute DNA with 5/15 ml Buffer QF. Collect the eluate in a 10 ml or
30 ml tube. Do not use polycarbonate tubes because they are not
resistant to the alcohol used in subsequent steps.
- Precipitate DNA by adding 3.5/10.5 (0.7 volumes) room temperature
isopropanol to the eluted DNA. Mix and centrifuge immediately at
 for 30 min
for 30 min  . Carefully decant the
supernatant. Mark the outside of the tube before centrifugation to
locate pellet more easily.
. Carefully decant the
supernatant. Mark the outside of the tube before centrifugation to
locate pellet more easily.
- Wash DNA pellet with 2/5 ml of room temperature
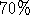 ethanol,
and centrifuge at
ethanol,
and centrifuge at  for 10 min. Carefully decant
the supernatant without disturbing the pellet.
for 10 min. Carefully decant
the supernatant without disturbing the pellet.
- Air-dry the pellet for 5-10 min, and redissolve the DNA in a
suitable volume of buffer (e.g. TE, pH 8.0, or 10 mM Tris-Cl, pH 8.5).
Redissolve the DNA pellet by rinsing the walls to recover all the DNA.
Do not pipet the DNA up and down, to avoid sheering.




Next: Protocol: Making Plates
Up: Protocol: Prep to Extract
Previous: Qiagen-tip 20 Miniprep
Ron Weiss
Wed Feb 10 15:48:08 EST 1999