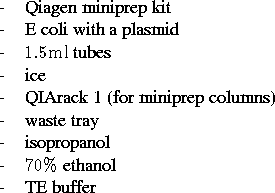Next: Qiagen-tip 100 Midiprep or
Up: Protocol: Prep to Extract
Previous: Protocol: Prep to Extract
Materials:
High copy number plasmid:
Methods:
- Centrifuge a
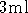 LB culture for 12 minutes. Decant the
supernatant. Resuspend the bacterial pellet in
LB culture for 12 minutes. Decant the
supernatant. Resuspend the bacterial pellet in 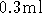 of Buffer
P1. Resuspend completely, and do not leave any cell clumps. Also,
chill buffer P3 on ice.
of Buffer
P1. Resuspend completely, and do not leave any cell clumps. Also,
chill buffer P3 on ice.
- Add
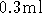 of Buffer P2. Mix gently, but thoroughly, by
inverting the tube 4-6 times (do not vortex). The lysate should
appear viscous. Close bottle for Buffer P2 immediately. Incubate
tube at room temperature for 5 min.
of Buffer P2. Mix gently, but thoroughly, by
inverting the tube 4-6 times (do not vortex). The lysate should
appear viscous. Close bottle for Buffer P2 immediately. Incubate
tube at room temperature for 5 min.
- Add
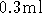 of chilled buffer P3, mix immediately but
gently by inverting tube 4-6 times, and incubate on ice for 5
minutes. The solution should become cloudy and very viscous.
of chilled buffer P3, mix immediately but
gently by inverting tube 4-6 times, and incubate on ice for 5
minutes. The solution should become cloudy and very viscous.
- Centrifuge at maximum speed (in a microfuge,
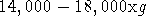 ), for 10 minutes. After centrifugation, supernatant should
be clear. If not clear, recentrifuge for a short amount of time.
Remove supernatant promptly.
), for 10 minutes. After centrifugation, supernatant should
be clear. If not clear, recentrifuge for a short amount of time.
Remove supernatant promptly.
- A few minutes before the centrifugation is over, place
QIAGEN-tips into a QIArack 1 over the waste tray. Equilibrate a
QIAGEN-tip 20 by applying 1 ml Buffer QBT, and allow the column to
empty completely by gravity flow. The resin bed will retain some
buffer, and will not readily dry out, of the tips can be left
unattended.
- Apply the supernatant from centrifugation to the QIAGEN-tip 20
and allow it to enter the resin by gravity flow. The supernatant
should be loaded promptly after centrifugation.
- Wash the QIAGEN-tip with
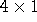 ml Buffer QC, using
gravity flow.
ml Buffer QC, using
gravity flow.
- Place the upper part of a QIArack 1 over the lower rack fitted
with clean 1.5 ml microfuge tubes. Elute DNA with 0.8 ml Buffer
QF using gravity flow, and collect the samples into the 1.5 ml
tubes.
- Precipitate DNA with 0.7 volumes (0.56 ml per 0.8 ml of
elution volume) of room temperature isopropanol. Mark the outside of
the tubes before centrifugation to aid in locating the resultant clear
pellet. Centrifuge immediately at maximum speed for 30 minutes in a
microfuge. Carefully decant the supernatant.
- Wash the DNA pellet briefly in
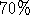 ethanol, centrifuge for 5
minutes, and decant the ethanol carefully. Air-dry for not more than
5 minutes. Redissolve in appropriate volume of TE buffer (e.g.
ethanol, centrifuge for 5
minutes, and decant the ethanol carefully. Air-dry for not more than
5 minutes. Redissolve in appropriate volume of TE buffer (e.g.  ) by rinsing the walls of the tube with buffer, but do not
pipet up and down.
) by rinsing the walls of the tube with buffer, but do not
pipet up and down.
- To determine yield, DNA concentration should be determined by
both UV spectrophotometry and quantitative analysis on agarose gel.
Change in methods for low-to-medium copy number plasmid:
Methods:
- Centrifuge a
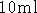 LB culture for 12 minutes. Resuspend
the bacterial pellet in an appropriate volume of Buffer P1. Use 0.3
ml of P1 for every 3 ml of LB culture.
LB culture for 12 minutes. Resuspend
the bacterial pellet in an appropriate volume of Buffer P1. Use 0.3
ml of P1 for every 3 ml of LB culture.
- Increase the volume of Buffers P2 and P3 to match the volume of
P1 used in the previous step.
- Centrifuge at maximum speed for 10 min at room temperature.
Transfer the supernatant promptly to a fresh tube and recentrifuge for
10 min at maximum speed. Transfer the supernatant to a fresh tube.
After centrifugation, the supernatant should be clear.
- Precipitate the DNA in the supernatant with 0.7 volumes room
temperature isopropanol. Centrifuge at maximum speed for 30 min at
room temperature and carefully decant the supernatant.
- Redissolve the DNA pellet in a small volume of TE, pH 7.0, and
add buffer QBT to obtain a final volume of at least 1 ml. Apply the
sample to a previously equilibrated QIAGEN tip 20.
- Continue with the standard purification protocol at step 7.




Next: Qiagen-tip 100 Midiprep or
Up: Protocol: Prep to Extract
Previous: Protocol: Prep to Extract
Ron Weiss
Wed Feb 10 15:48:08 EST 1999