Developing an appropriate disease model requires two major steps, deciding what diseases need to be included and determining the causal structure among the diseases, intermediate states, and findings. The domain is management of the hemodynamic consequences of heart failure. Therefore we need to include the diseases that alter the hemodynamics. However, there are many definitions of a disease. One important disease of the myocardium is congestive cardiomyopathy. This disease can be further classified by causes (alcohol, sarcoidosis, idiopathic, etc.) but no matter what the cause, the effect on the cardiovascular hemodynamics is the same. That is not to say that the entire treatment is the same - if there is a treatable cause weakening the heart, that must be treated - but those aspects involved in correcting the hemodynamic dysfunction remain the same. Therefore, we have one node for congestive cardiomyopathy and it is considered a primary cause. Since there is also a node for ischemic heart disease in the model which can cause congestive cardiomyopathy, that causal link must be included. Thus, congestive cardiomyopathy may be primary or caused by other nodes. The decision about what to include is not always so simple. There is an uncommon disease, left atrial myxoma, that partially obstructs the mitral valve and produces the same hemodynamic dysfunction as mitral stenosis. The physical examination clues to distinguish the two diseases are quite subtle, but if either disease were suspected, an echocardiogram or other imaging test would be done which would clarify the situation. Therefore, there is no advantage to including the less common disease and the common disease node is used to cover both. There are also a number of congenital diseases that have been left out of the program because such diseases are not initially found in the adult (our target population). Thus the model has in it the cardiovascular causes of the hemodynamic dysfunctions at the level needed to distinguish the different hemodynamic manifestations.
There are also a number of diseases with findings similar to those produced by hemodynamic dysfunction. For example, the pulmonary findings of pneumonia are often very difficult to distinguish from those of cardiac pulmonary congestion. Since such diseases are often the most important differential diagnoses, they must be included. The problem again is the level of detail. Both low cardiac output and renal insufficiency cause retention of fluids, but there are many different kinds of renal insufficiency because there are many kinds of kidney disease. Our solution has been to only cover such diseases to the extent that they produce findings similar to cardiac findings. Thus, we have entities such as renal insufficiency and primary liver disease. The result is a model with decreasing specificity as one gets away from the central focus of hemodynamic disorders.
Developing the model requires a change of perspective for the cardiologist expert. Usually the cardiologist thinks in terms of diseases and findings and not in terms of the intermediate causal mechanisms. To develop a model that includes the intermediate nodes, we started with lists of associations between diseases and findings, identified findings generated by the same mechanisms, and added intermediate nodes representing those mechanisms. For example, many diseases cause shortness of breath, rales (noises in the lungs), and certain X-ray findings. These are all produced by fluid buildup in the lungs, so an intermediate node was added for pulmonary congestion. Identifying intermediate nodes was not done blindly. We started with the model used in the program to predict the effects of therapy[Long88]. This model has equations relating the constraints among parameters and dependencies on physiologic state. Since diagnosis of the patient provides much of the information needed to establish the pathophysiologic state for this model, the intermediate nodes must be compatible.
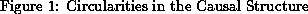
The use of the predictive model also pointed out situations requiring circularities in the causal structure. Two circularities are shown in Figure 1. In both cases low cardiac output causes a high sympathetic state. The high sympathetic state increases the heart rate, but a high heart rate can decrease cardiac output, especially in situations such as aortic stenosis, when ejection time is prolonged. The high sympathetic state can also increase the systemic vascular resistance, which increases blood pressure and the load on the heart, which decreases left ventricular emptying and cardiac output. Both of these situations are positive feedback loops. If any of the nodes is triggered by some pathophysiological situation, there is a significant probability that the succeeding nodes in the loops will result. Indeed, one reason that therapies to decrease heart rate or decrease vascular resistance are often effective in patients having low cardiac output is that these mechanisms often overdo their jobs.
Using the therapy model and clustering the nodes by physiologic mechanism, we built a model that covers the pathophysiology of cardiovascular hemodynamics. The resulting model has as many as a dozen intermediate nodes between a primary node and a terminal node. It makes explicit the mechanisms that produce the findings and allows the representation to distinguish the variations and combinations of diseases and alterations due to therapies. Because we started with the list of associations, we also have that as a check of the expanded model. That is, we can compare the expected associations in the expanded model to our initial list of associations and use the differences to refine the model.