This report examines the capabilities of a new approach to medical decision support systems. The approach makes use of a causal physiological model for relating clinical and laboratory data to the mechanisms responsible for the patient's disorder and provides methods to aid the user in reasoning from that model about diagnostic and therapeutic questions. The model and methods are accessible, allowing one to use the program as a reasoning blackboard to examine the implications of hypotheses and possible therapies. We are applying the approach to the diagnosis and management of severe heart failure and also to the management of angina. The program is still in active development at a stage where it is capable of reasoning support of the type discussed in this report.
Many of the manifestations of heart failure are the result of mechanisms which attempt to compensate for low cardiac output. Determination of the cause of a patient's heart failure and of any important complications requires the interpretation of observations in light of the underlying mechanisms. Our approach uses a partially constrained physiological model to represent the state of the patient. The user has access to this model through procedures to enter data, do diagnostic and therapeutic reasoning, and obtain explanations of the patient state. Ultimately we shall develop a program in which the user can enter what he or she knows about a given patient, review the implications of that information in terms of what must or must not be true of the physiological state, consider the implications of hypotheses accounting for that state, look for strategies for gathering appropriate clarifying data, and consider possible therapies. This program might be characterized as a reasoning blackboard for thinking about a patient with cardiovascular disease.
Our intent is to develop a program for helping physicians reason about the diagnosis and management of patients with severe or complex heart disease of any etiology. The program is in an early stage of development. To simplify the process of developing the representational methodology, we have recently developed a more restricted physiological model for the management of angina, including patients whose cardiac performance is marginal. (The heart failure program and angina program only differ in the model.) The models represent information about the nature of the etiologies, causal relationships, therapies, and measurements. This report provides examples of the current capabilities of the angina program.
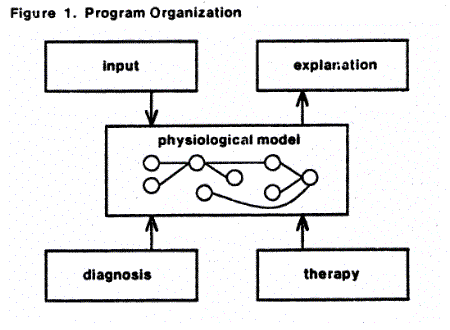
The input module receives the data about the patient and sets the qualitative parameters in the physiological model. The other modules operate from the physiological model to assess the completeness of the diagnosis and plan ways of improving it; to search for possible therapeutic measures and to anticipate their possible effects, and to explain the model to the user. (The organization of the program is discussed in greater detail in an earlier paper.(long))
The physiological model is central to the program structure. It is a network of nodes representing qualitative values of physiological parameters. For example, there are nodes representing angina and another representing high heart rate. All nodes initially have the value unknown, reflecting the initial state of knowledge about the patient. As the input module acquires and assesses data, some nodes are assigned truth values, either when the evidence is sufficiently strong for the program to make that conclusion directly or when the user decides the value is appropriate. These nodes are connected by a network representing the minimal logical constraints that must exist among them. Thus, angina implies inadequate myocardial oxygen supply. Logical relations are automatically maintained by a Truth Maintenance System by propagating the implications of each assertion (either true or false)(McAllester). Thus, the strongest physiological relationships ensure consistency in the model. When an assertion is not consistent with the current state of the model, the inconsistencies are presented so the user can withdraw any that are not appropriate; the system will reconfigure the logical implications appropriately. Conclusions based on less dependable reasoning are encoded as heuristics under the user's control, or he or she can add hypotheses to the model.
The nodes of the physiological model are selected to represent clinically distinct qualitative values of physiological parameters. That is, each node represents a range of values with a potential qualitative impact on patient management. This design permits us to factor the reasoning task into two components: (1) interpreting patient data to determine the truth or falsity of various nodes, and (2) determining what diseases and therapies are consistent with the known nodes. Quantitative information is not discarded. Rather, it is accessible for program use in gathering input, making diagnoses, and recommending therapy. The nodes represent physiological parameters, primary causes, and therapies. Thus, cardiac performance, coronary artery disease, and nitroglycerin therapy are all parameters of the model. Therapies are included because diagnosis is not a one-time determination, but is intertwined with patient management. A disease is represented by the chain of abnormal nodes implied by the various observations about the patient. (This is similar to the disease representation in CASNET except our model represents physiological causation while CASNET represents the history of the disease.(weiss)) Thus, our representation can handle multiple diseases and multiple presentations of disease as additions and variations to these chains of nodes. The intent is to distinguish disease states that would change the therapy needs.
In addition to the links with possible causes and effects, nodes include links to the possible therapy nodes, an importance flag to focus the diagnosis and therapy, and a list of possible measurements that might provide evidence for the truth of the node. The measurements have evaluation functions that determine the evidence provided by measurement values. Both the therapy nodes and measurements have risks, benefits, and requirements that translate into costs to aid in selection. An example node is in figure 5.
Thus, the model records the evolving interpretation of the state of the patient. It also is a data structure from which diagnostic and therapeutic reasoning is done. Procedures can examine the relationships in the model and plan diagnostic investigations to increase the knowledge about the causes of the patient's manifestations or look for possible therapeutic interventions to interrupt the causal chains from the primary causes to the dangerous effects.
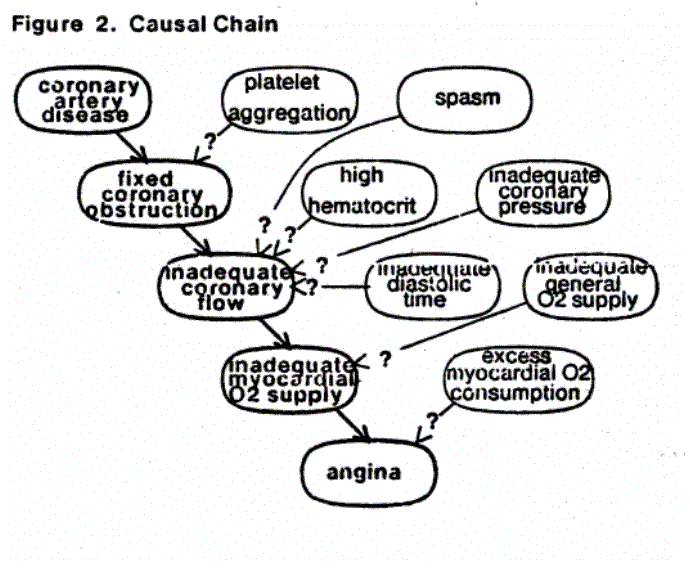
Still, there are several aspects of the patient's condition that are not known. Fixed coronary obstruction could be worsened by platelet aggregation. Inadequate coronary flow could be aggravated by some component of inappropriately increased coronary tone (spasm), inadequate perfusion pressure, high hematocrit, or inadequate diastolic time. Inadequate myocardial oxygen supply due to inadequate coronary flow could be worsened by further reduction in respiratory oxygen delivery.
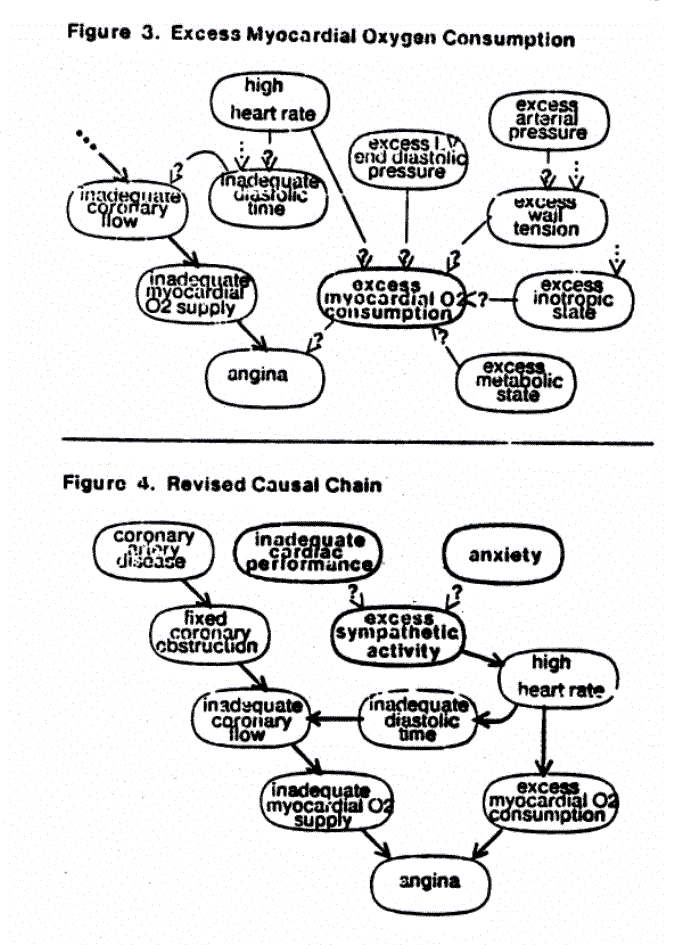
Finally, the angina could be worsened by a number of factors which act to increase the myocardial oxygen consumption. The part of the model relating to the oxygen consumption is shown in figure 3. When the diagnostic module is consulted, it searches the nodes that might affect the known causal chain to find measurements that may clarify the situation. The difficulty of measuring the parameters represented by these nodes varies considerably --- the heart rate is determined simply by taking the pulse, whereas the inotropic state is measurable only with complex techniques requiring cardiac catheterization.
In making its recommendations, the diagnostic module assesses the cost and risk of the measurements to select ones that could clarify the diagnosis at minimum risk. In this example no invasive procedures have been reported. The program makes several recommendations, two of which will help determine the myocardial oxygen consumption: the heart rate (both for its effect on oxygen consumption and the influence the diastolic interval has on coronary flow) and blood pressure (for information on wall tension, diastolic pressure, and stroke volume). These will only clarify some relations in the model, but that may be sufficient to consider therapy.
Entering the information that the heart rate is 90, the blood pressure
is 130/80, and the other requested measurements (including an ECG) are
normal, the causal chain changes to that summarized in figure 4.

Thus, the diagnostic module has concluded that a significant factor in this patient's angina is the high heart rate. If there is no primary tachyarrhythmia responsible (and the ECG was normal), the cause must be excess sympathetic activity, assuming that the causal knowledge base is complete. (The program can point out deductions that need collaborative evidence.) The blood pressure, on the other hand, might or might not have been too high, depending on the patient. In this case, the user chose to leave the excess arterial pressure node unknown. The new data about heart rate and blood pressure shed light on the patient's condition, but no cause is yet known for the excess sympathetic activity. Looking at this node reveals the following (caused-by lists etiologies in two groups P+: possible causes and W+: worsening or precipitating factors):
NOW: (EXCESS SYMPATHETIC-ACTIVITY)
caused-by P+ (INADEQUATE CARDIAC-PERFORMANCE) = UNKNOWN
HYPERTHYROIDISM = FALSE
(INADEQUATE ARTERIAL-PRESSURE) = FALSE
ANXIETY = UNKNOWN
Two possible causes for excess sympathetic activity
not yet eliminated are anxiety and inadequate cardiac performance.
Looking at inadequate cardiac performance shows:
(INADEQUATE CARDIAC-PERFORMANCE) = UNKNOWN
-- important in its own right
caused-by P+ (INADEQUATE STROKE-VOLUME) = UNKNOWN
W+ (INADEQUATE HEART-RATE) = FALSE
measures FATIGUE = FALSE
COOL-AND-CLAMMY-SKIN = unspecified
OLIGURIA = unspecified
CARDIAC-INDEX = unspecified
-- MODERATE evidence for FALSE
effects P+ (INADEQUATE ARTERIAL-PRESSURE) = FALSE
(EXCESS LAP) = UNKNOWN
(EXCESS SYMPATHETIC-ACTIVITY) = TRUE
Thus, the program considers the absence of fatigue moderate evidence that the cardiac performance is not too low, but there are a number of measurements as yet unspecified that should be considered before inadequate cardiac performance is excluded, because the importance property indicates it would be another focus for therapy if it is present.
Similarly, the user could look at anxiety, which is more difficult to measure. Assuming the user decides the inadequate cardiac performance node is false (which the user can assert directly), anxiety becomes the explanation for the high heart rate. With a complete plausible causal interpretation of the findings, the user and the program have made an initial diagnosis.
Therapy for (INADEQUATE CORONARY-FLOW) is NITROGLYCERIN Therapy for (HIGH HEART-RATE) is BETA-BLOCKER Therapy for ANXIETY is SEDATIVEThe program can now speculate on the likely effects of these therapies on the patient. Nitroglycerin will increase the coronary flow*
Another possibility that would have been suggested, if the arterial pressure were concluded to be too high, is an arterial vasodilator. Even though the program did not suggest this therapy, the user could ask the program to speculate on the effects. Decreasing the arterial pressure would certainly decrease the wall tension and the myocardial oxygen consumption, but it would also decrease the coronary perfusion pressure and might decrease the coronary blood flow. With these opposing changes, it is not clear whether the angina would be improved, made worse, or not changed.
The program assesses the possible beta blocker therapy by following the causal links in the model as follows:
Possible effects of BETA-BLOCKER:
may cause (INADEQUATE HEART-RATE) - unlikely, but watch
may cause (INADEQUATE INOTROPIC-STATE) - need more data
(UNKNOWN INOTROPIC-STATE): moderate decrease
-- this may cause a decrease in CARDIAC-PERFORMANCE
(HIGH HEART-RATE): moderate decrease
(EXCESS MYOCARDIAL-O2-CONSUMPTION): moderate decrease
(INADEQUATE DIASTOLIC-TIME): moderate increase
(INADEQUATE MYOCARDIAL-O2-SUPPLY): moderate increase
ANGINA: large decrease
Thus, while the beta blocker may cause a decrease in the inotropic
state and could produce adverse effects if the contractility were
marginal, the beta blocker might have two beneficial effects ---
decreasing the oxygen consumption (by decreasing the heart rate) and
increasing the oxygen supply (by increasing the diastolic time).
Thus, the program has provided a starting point for reasoning about therapy in this patient, even though there are many things not yet known about the patient's condition.
After the patient is treated, there will be more information to refine the diagnosis and therapy. Consider the situation in which angina persists after the heart rate has been lowered by a beta blocker. In that case, one therapeutic alternative is to decrease the arterial pressure (with no assurance that the angina will be helped). On the other hand, an invasive diagnostic procedure --- a pulmonary artery line --- will determine the LAP. The program can point out that the same procedure will determine the cardiac output and enable intravenous therapy. These data could help to determine the appropriate next therapeutic maneuver.
The program is able to use the relative risks and benefits of diagnostic information and therapeutic maneuvers in making suggestions. Prior to the initial therapeutic trial of a beta blocker there was no compelling reason to ask for the LAP. The procedure is invasive and hence risky. Also, other information was available. The relative risks also eliminated several therapies. Since it was concluded that there must be some degree of coronary obstruction, angioplasty is a possible therapy. However, that is considerably more costly and risky and not justifiable until other avenues have been exhausted.
The program also can focus on the dilemma of refining the diagnosis versus proceeding with therapy. When it was determined that the patient had a rapid heart rate, the need to further refine the diagnosis was greatly diminished because there was a likely cause for which there was a simple therapy. The most appropriate response was to try the therapy. However, once it had been determined that the therapy failed to achieve the desired control of the angina, further refinement of the diagnosis was needed. At that point it was reasonable to suggest an invasive procedure to determine the LAP, the cardiac output, and enable intravenous therapy. If LAP were high, both oxygen supply and consumption would be improved by decreasing it. Thus, the response to the therapy can be used to further refine the diagnosis.
The capabilities of this program depend on two factors: having the appropriate kinds of information available for making decisions and having the flexibility to assist the user in reasoning wherever the input and user direct that reasoning. The information for making decisions is highly organized by the nodes in the physiological model. The causes, effects, therapies, measurements, and importance provide a rich knowledge base enabling both the program and the user to make appropriate decisions. The flexibility of reasoning depends on a sharing of responsibilities between the program and the user. The program makes deductions strongly supported by the data, asks the user about deductions for which there is significant evidence, and waits about nodes with little or no evidence. The program provides guidance by maintaining and exploring the causal chains established in the patient. Thus, the program can focus attention on the nodes that will determine the appropriate therapy. The user can provide direction by making or suspending decisions, entering new data, or simply adding or withdrawing specific assertions.
The approach we are developing for physiologically based reasoning has capabilities that should make it an effective tool for the physician caring for a specific patient as well as an educational tool for developing appropriate clinical reflexes and judgments.